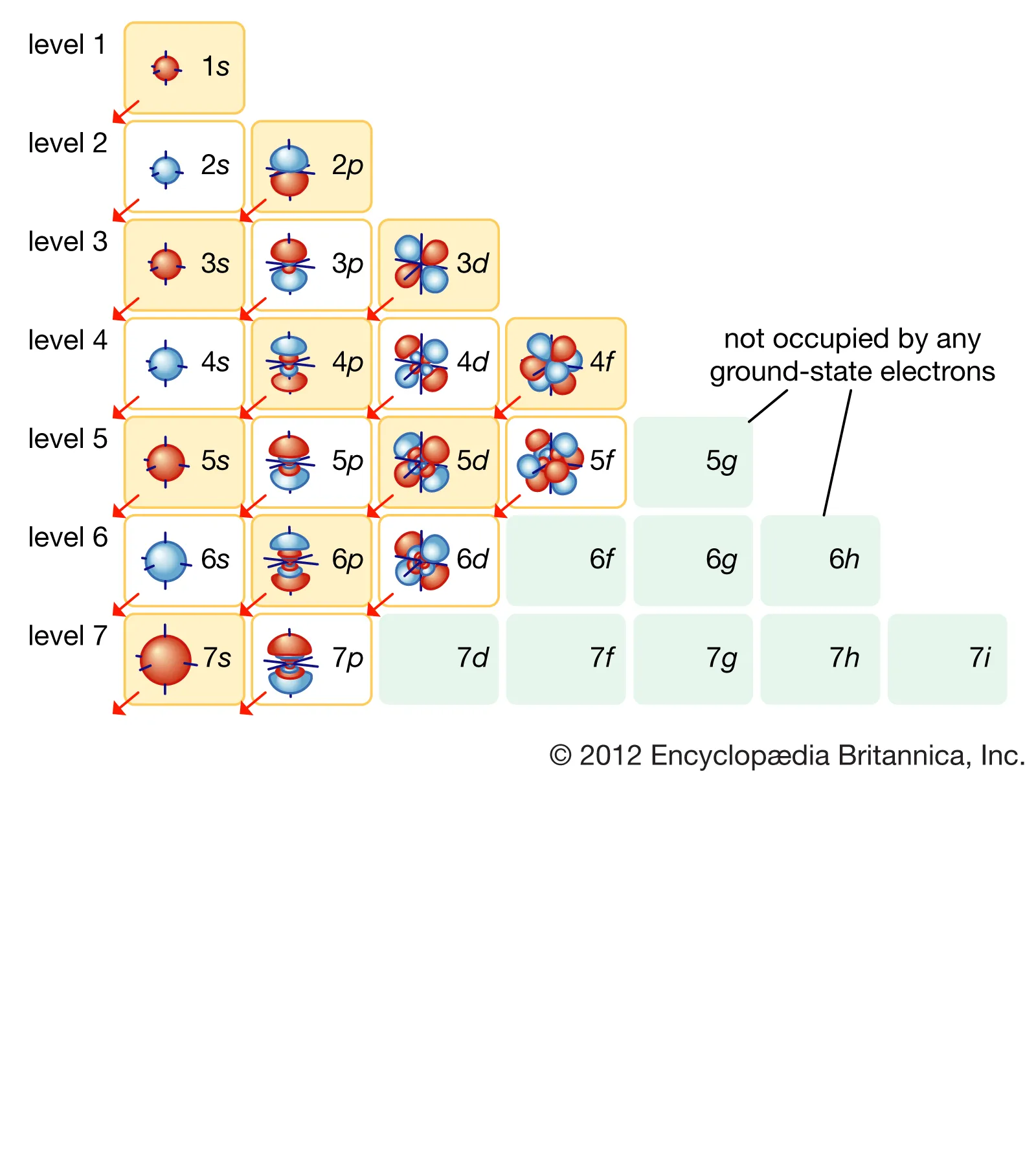
As electron states gain a higher quantum number the orbital reaches farther away from the element's nucleus, as can be seen from the graphed orbitals on this page. From s to p to d to f the orbitals increase in complexity. Note that the orbitals shown in the illustration are maps of places the electrons inhabit, but that the mapped regions are not the electrons. For an orbital that is full, the electrons filling the orbital are said to be degenerate, meaning all have the same energies. In a valence orbital, electrons may be found in different parts of the mapped clouds (pictured to the left), and they will have different angular momentums, giving them different energy levels. There are four quantum (spatialized) numbers to locate an electron, when all four (n, l, ml, ms) are completely observed. n is the shell of the orbital (s, p, d, f), l is the subshell, l being the region of the shell (we can see p having three l numbers when n is three), ml is the orientation of the orbitals, the value for n - three of the p orbital it would be five. The final quantum number (spin) ms is only observable if an electron is exposed to a magnetic field and if it spins with the field it is called 1/2 and if it spins against the magnetic field it is called -1/2. -1/2 has a slightly higher energy than 1/2. WORKS CITED- Encyclopedia Britannica, Chegg (Orbital Levels Map) Chemistry LibreTexts, Flowers Paul (2019). Accessed July Thirteenth, 2022. Address: https://chem.libretexts.org/Courses/Oregon_Institute_of_Technology/OIT%3A_CHE_202_-_General_Chemistry_II/Unit_2%3A_Electrons_in_Atoms/2.2%3A_Atomic_Orbitals_and_Quantum_Numbers#:~:text=Generally%20speaking%2C%20the%20energy%20of,location%20of%20the%20energy%20level.